IS 516 [Part 5/Sec 3] – 2021
Principle
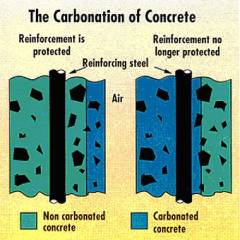
Carbon dioxide from air reacts with the calcium hydroxide in concrete to form calcium carbonate which reduce the alkalinity of the concrete and increase the risk of reinforcement corrosion.
Carbonation of concrete is a slow and continuous process progressing from the outer surface inward, but slows down with increasing diffusion depth.
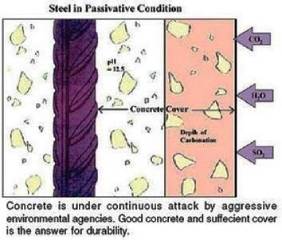
Cement paste contains 25-50 wt% calcium hydroxide (Ca(OH)2), which mean that the pH of the fresh cement paste is at least 12.5. The pH of a fully carbonated paste is about 7.
The concrete will carbonate if CO2 from air or from water enters the concrete according to:
Ca(OH)2 + CO2 —> CaCO3 + H2O
When Ca(OH)2 is removed from the paste hydrated CSH will liberate CaO which will also carbonate. The rate of carbonation depends on porosity & moisture content of the concrete.
The carbonation process requires the presence of water because CO2 dissolves in water forming H2CO3. If the concrete is too dry (RH <40%) CO2 cannot dissolve and no carbonation occurs. If on the other hand it is too wet (RH >90%) CO2 cannot enter the concrete and the concrete will not carbonate. Optimal conditions for carbonation occur at a RH of 50% (range 40-90%).
Carbonation has three effects: it increases mechanical strength of the carbonated concrete, reduces permeability and it also decreases alkalinity, which is essential for corrosion prevention of the reinforcement steel. Reinforcement in concrete will not corrode, if the pH of concrete is maintained around 13. Carbonation reduces the pH level of concrete. Below a pH of 10-11, the steel’s thin layer of surface passivation dissolves and corrosion is promoted. The pH of carbonated concrete drops to about 7, thus, carbonation provides a favourable condition for corrosion. The depth of carbonation increases with an increase in water / cement ratio. It is to be noted that corrosion of reinforcement will start if entire cover to the steel is carbonated , but presence of moisture and oxygen is essential.
Carbonation rate is normally high in dry weather, but possibility of corrosion is less due less moisture content
Procedure
The extent / depth of carbonation is determined by treating freshly broken / core cut concrete surface or a drilled powder obtained from various depths, with a phenolphthalein indicator. A purple red colouration will be obtained where the highly alkaline concrete has been unaffected, but carbonated portion will remain uncoloured. The change of colour corresponds to a pH of about 8.3.


Colourless Concrete is a Carbonated Concrete
